Using Nanomaterials to Purify Water
Researchers’ invention could do the work of a water treatment plant on a small scale
Two UTSA professors are working on a new light-activated material that can do the work of a water treatment plant. Heather Shipley, chair of the Department of Civil and Environmental Engineering and the Burzik Professor in Engineering Design at UTSA, and Kelly Nash, associate professor of physics, have received a $65,000 grant from the National Science Foundation to develop a nanomaterial that can do the work of a water treatment plant.
Shipley’s main area of focus is water, and in previous research projects she has worked with nanomaterials that are commercially available. Because Nash’s expertise is nanomaterials, the two colleagues chose to collaborate in making a composite of several nanomaterials so that one material could do the work of many.
“We’re working to create a nanocomposite material to treat pollutants in water,” Shipley said. “This could be used for in-home water treatment, or it could be used in developing countries where the infrastructure for water treatment plants might not exist.”
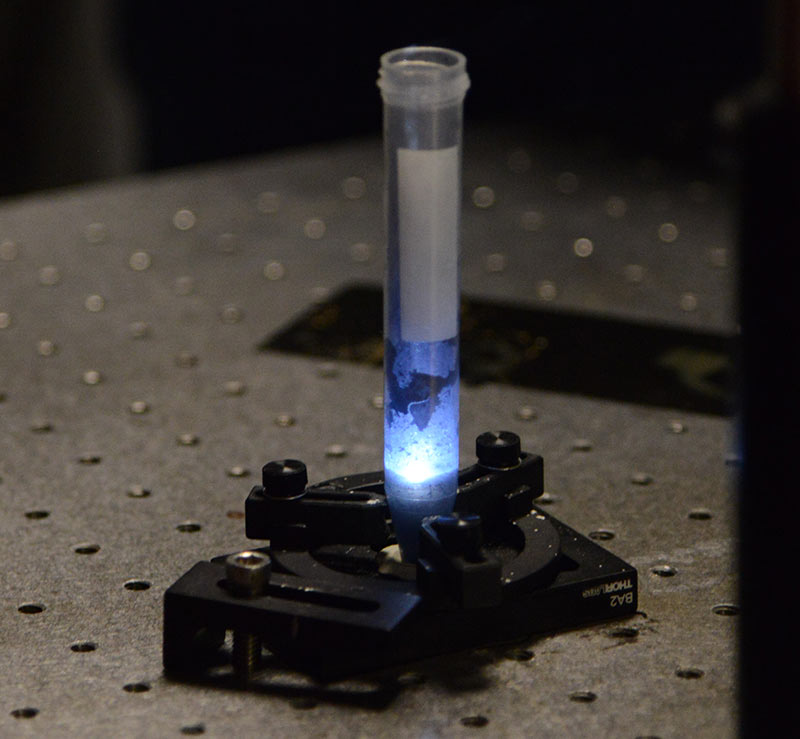
Nanomaterials are chemical substances or materials that are manufactured and used at a very small scale. They are developed to exhibit novel characteristics compared to the same material without nanoscale features, such as increased strength, chemical reactivity or conductivity. The material that Shipley and Nash are creating is entirely new. It’s activated by sunlight, causing organic pollutants to be removed through light reaction and heavy metals to be attached to the material and removed from water.
“It’s doing the job of a water treatment plant, but it also goes a step further,” Nash said. “In a plant, there are many processes to catch these pollutants, but when you get down into the levels of microbial and toxic metal ions, you need a nanomaterial to filter them.”
Shipley noted that water sources all over the world are polluted by industrial processes and in various other ways, which makes the water hazardous to use. Rather than focus on one specific pollutant, she and Nash wanted to create a material that could address a majority of them.
“It made sense to create an end-user type of solution instead of a new type of water treatment plant for a new city,” Shipley said. “This way, it’s accessible and much more feasible, especially in developing areas where there’s minimal water treatment.”
Shipley and Nash have now been developing the new material and are satisfied so far with its performance but are now facing a new challenge: making the material reusable.
“Once it’s activated by light, it does what it’s been designed to do, but then it’s done,” Nash said. “We don’t want to make more waste, so now we’re working on making the material regenerate so it can be used again and again.”
—Photos by Deborah Silliman/College of Engineering